Hi everyone, welcome back to another blog post!!
This is my first post of 2024 as I have been focusing on my training the laboratory and doing a lot of outreach talks.
I do hope you all enjoy this post, so sit back, grab a cuppa and happy reading!!
What is Myeloma?
Myeloma, also known as multiple myeloma is the malignancy of plasma cells produced in the bone marrow. It is defined as the proliferation (rapid production) of a clone of plasma cells in the bone marrow.
The reproduction of plasma cells are limited by homeostasis, this means it only produces the required amount of immunoglobulins for that “time” for example to fight or eliminate an infection.
However, on the occasion a plasma cell can escape from this control and reproduce itself millions of times over and over. The continuously replicated cells also secretes its “programmed” Immunoglobulin isotype (IgG, IgM, IgA, IgD and IgE). These isotypes are produced in such large amounts that it can be seen on electrophoresis.
What are immunoglobulins?
Immunoglobulins are a family of proteins of the humoral immune system and made up of five isotypes. They are composed of two types of polypeptide chains: heavy chains and light chains. The light chains are further classified into two types; kappa (κ) and lambda (λ) chain.

Epidemiology of Myeloma:
Myeloma is a known disease of the elderly, however, it is a disease that can impact those below the age of 65. In the UK they are approximately 24,000 people living with myeloma and around 5900 people are diagnosed yearly.
Pathogenesis of Myeloma:
The proliferation of plasma cells stimulates growth factors released, this may impact nearby osteoclast (cells that degrade bone to initiate bone remodelling) which in turn suppresses osteoblast inhibiting bone formation, this leads to lytic or osteolytic lesions. These lesions appear as small holes in the bone which can lead to possible bone fractures. This also causes more calcium to be released and the patient to present high calcium levels. Most of the skeleton is impacted including the long bones.
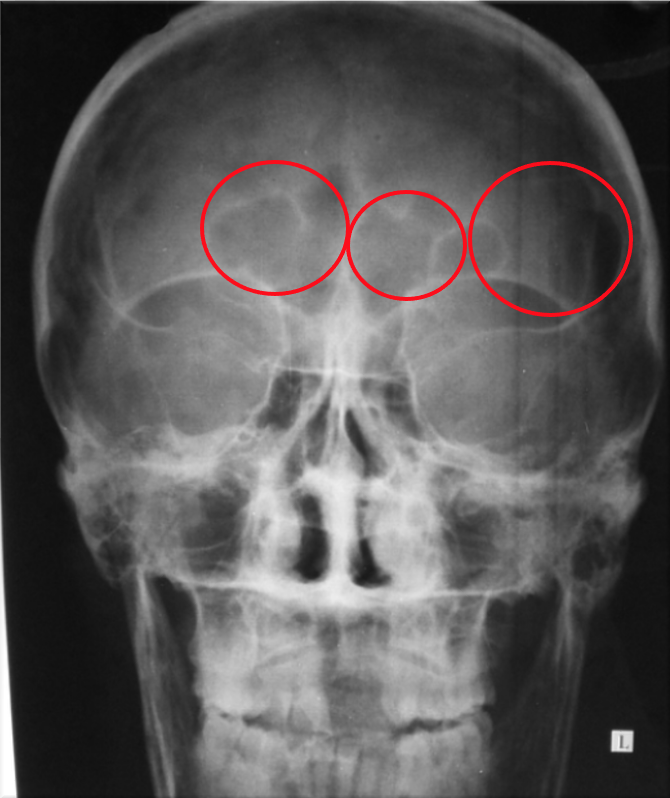
Proliferation of plasma cells also decreases the bone marrows ability to produce red blood cells leading to the formation of anaemia. In myeloma due to the excess immunoglobulin produced, excess free light chains are also deposited. Due to their low molecular weight they can enter the kidney and pass through the glomerulus to the distal tubule, blocking the tubule leading to renal failure.
Symptoms:
Patients usually show symptoms of shortness of breath, palpitations and weakness which is linked to anaemia.
They will also feel extreme thirst, sick and the need to urinate frequently due to the raised calcium levels. Signs of renal failure is usually associated with weight loss, poor appetite, swollen ankles, feet or hands and hiccups that won’t go away.
Bone fractures and compression of spinal cord due to spinal fractures can cause pins and needles, numbness and weakness in the legs and feet and problems controlling your bladder and bowel.
How do we test for myeloma in the Immunology laboratory?
The patient’s sample is first tested on the BNII Nephelometry. This analyser measures the levels of immunochemical protein in serum using light scattered by antigen and antibody completed formed. The unknown value is measured against a generated reference curve where the volume of both antigen and antibody is known and this calculates the level of antigen in the serum.

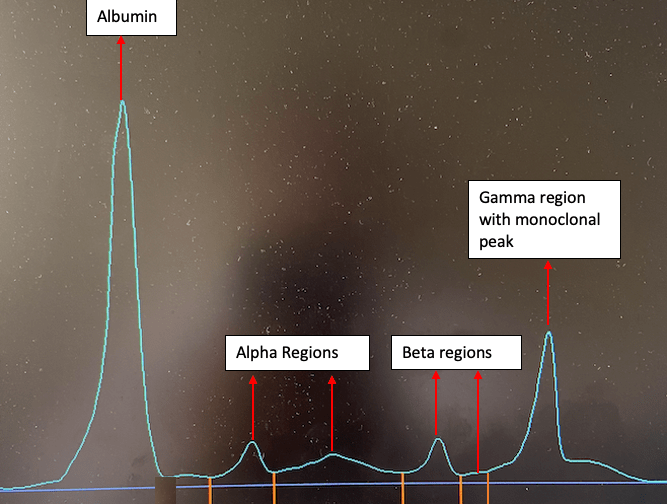
After the sample has been run on the BNII, it will be tested on the capillary which uses zone electrophoresis, this technique is known as serum protein electrophoresis. The principle of this technique is based on the separation of protein based on their charge. This produces an electrogram which can be seen in Figure 4.
Serum protein electrophoresis provides an overview of the patients serum protein pattern and can show any protein abnormalities. Some examples of protein abnormalities which are detected are:
- Alpha 1 antitrypsin deficiency
- IgG deficiency
- Raised Alpha 1 and Alpha 2 globulin
- Polyclonal raised gamma
- Presence of a monoclonal protein
If the isotope being produced is a product of a single clone it is referred to as a monoclonal immunoglobulin and if it’s a product of different clones of plasma B cell, it is referred to as a polyclonal immunoglobulins.
Following up from the serum protein electrophoresis, if there’s a monoclonal protein present an immunofixation will be carried out. The main purpose of immunofixation is to identify the presence and determine the isotype of a monoclonal immunoglobulin in the patient’s serum. The principle of this technique is the use of gel electrophoresis followed by reaction with antisera against heavy and light chains. This technique is carried out on the Hydrasys.
Did you know immunofixation is the only way of determining the presence of a small monoclone under a electrophoretic band?
Case study:
A 76 year old patient presenting with weight loss, tiredness and back pain got their blood tested for a myeloma screen. Blood reports provided from Haematology and Biochemistry showed high calcium and anaemia. X-rays showed bone lesions and a back fracture.
Immunology results showed a very raised IgG.
IgG 36.4g/l (Reference range: 6-16g/l)
IgM 0.22g/l (Reference range: 0.2-8g/l)
IgA <0.07 g/l (Reference range 0.8-4g/l)
Thier serum free light chains were tested for and the results appeared to be normal.
SFLC Kappa: 21 mg/l (Reference range 6.6-22.4mg/l)
SFLC Lambda: 14mg/l (Reference range 8.3-27mg/l)
The sample was run for electrophoresis and a monoclonal peak had been detected in the gamma region.
Due to the presence of the monoclonal peak an immunofixation was carried out to identify the para protein present.
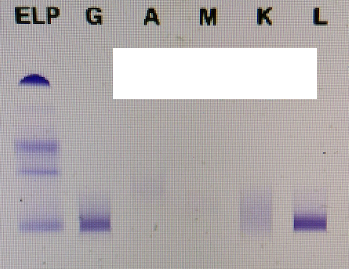
What happens next to our myeloma patient?
The patient is referred and assessed by haematology to discuss treatment options and whether they will be suitable for chemotherapy as the quality of life impacts some patient’s need for a bone marrow transplant. Chemotherapy is provided to the patient and they are treated with monoclonal antibody drugs, the two most common monoclonal antibody drugs are isatuximab and daratumumab.
Thank you so much for reading this post on Multiple Myeloma and I hope it gives you all a little insight on how the Immunology laboratory plays a role in the diagnosis of Multiple Myeloma.
References:
Bn II system (no date) BN II System. Available at: https://www.siemens-healthineers.com/en-uk/plasma-protein/systems/bn-ii-system (Accessed: 01 July 2024).
Kaur, S. (2023) Multiple myeloma: Radiology case, Radiopaedia. Available at: https://radiopaedia.org/cases/multiple-myeloma-19 (Accessed: 23 July 2024).
Multiple myeloma (no date) NHS choices. Available at: https://www.nhs.uk/conditions/multiple-myeloma/symptoms/ (Accessed: 20 July 2024).
Giuliani, N., Rizzoli, V. and Roodman, G.D. (2006) ‘Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition’, Blood, 108(13), pp. 3992–3996. doi:10.1182/blood-2006-05-026112.
Terpos, E. et al. (2018) ‘Pathogenesis of bone disease in multiple myeloma: From bench to bedside’, Blood Cancer Journal, 8(1). doi:10.1038/s41408-017-0037-4.
Leave a comment